Gas Laws
Important Questions on Gas Laws
For an ideal gas, Boyle's law is best described by -
The plot that is not valid for an ideal gas where is the pressure and is the volume of the gas is:
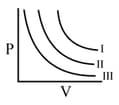
are three isotherms, respectively, at and as shown in the graph. The temperature will be in the order:
Assuming that the molecule is spherical with a radius of , the percentage of the volume of the molecules to the total volume of the gas at is
at contained in a flask was replaced by under identical conditions of pressure, temperature and volume. Then, the weight of will be ____ of .
The absolute zero is defined as the temperature
At constant volume, for a fixed number of moles of a gas, the pressure of the gas increases with the increase in temperature due to:
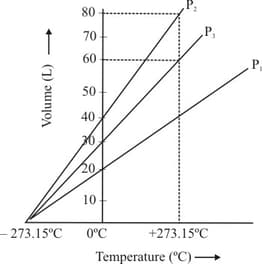
The volume-temperature graph of a given mass of an ideal gas at respective constant pressures is shown below. What is the CORRECT order of pressure?
If hydrogen and argon are kept in two separate but identical vessels at a constant temperature and pressure, which of the following is correct?
At constant and , Avogadro law is represented as _____
Mathematical expression of Gay-Lussac's law is given as _____.
On absolute temperature scale, is:
________ is absolute zero temperature. Which is correct option for blank space?
Which of the following expression for a given mass of a gas at constant pressure represents Charles law?
Which property is kept constant in the verification of Charles law?
Equal masses of and methane have been taken in a container of volume at temperature in identical conditions. The ratio of the volumes of gases methane would be:
moles of an ideal gas is present in a closed container at a pressure of . Which of the following is a correct graphical representation of it?
A vessel was filled with oxygen at and weighed. It was then evacuated and filled with gas at the same temperature and pressure. What will be the weight of oxygen?
For a given mass of a gas at constant temperature , if the volume becomes three-fourth, then the pressure will become:
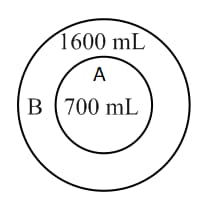
Two balloons, and are filled with a gas at . Maximum capacities of balloons, A and B are and , respectively. When the balloon system is heated, which one will burst first?

