Embibe Experts Solutions for Chapter: Solutions, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 2: Level 2
Attempt the practice questions on Chapter 8: Solutions, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 2: Level 2 with Hints & Solutions
Which one of the following statements is FALSE?
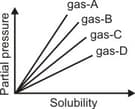
From the given graph at constant temperature, which gas has the least solubility?
To observe an elevation of boiling point of , the amount of solute to be added to of water () is _____.
Van't Hoff factor of centimolal solution of is . Calculate the percent dissociation of .
The freezing point of benzene decreases by when of acetic acid is added to of benzene. If acetic acid associates form a dimer in benzene, percentage association of acetic acid in benzene will be _____. ()
aqueous solution of urea is isotonic with _____.
An aqueous solution contains acetic acid and ethanol. The mole fraction of acetic acid ethanol and water in order is given by______.
If of a solute was dissolved in of water and osmotic pressure of the solution was found to be at , then molecular weight of the solute is ___ .
