Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Level 3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Level 3
Attempt the practice questions on Chapter 11: Surface Chemistry, Exercise 3: Level 3 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Level 3 with Hints & Solutions
Modified quantum dot nanoparticles_____
The pressure of the gas was found to decrease from to of Hg. When of the sample of activated charcoal was kept in a flask of one litre capacity maintained at . If the density of charcoal was at . The volume of gas adsorbed per of charcoal at of is:
Graph between and is a straight line inclined at an angle of When pressure is and the amount of solute adsorbed per of the adsorbent will be:
Which is an example of coagulation?
The adsorption isotherm for a gas is given by the relation , where is moles of gas adsorbed per gram of the adsorbent, is the pressure of the gas, and and are constants. Then, :
Statement : Micelles are formed by surfactant molecules above the critical micelle concentration () because
Statement : The conductivity of a solution having surfactant molecules decreases sharply at the .
Among the following, the surfactant that will form micelles in the aqueous solution at the lowest molar concentration at ambient conditions is
(Hint: During the micelle formation, the number of ions decrease as a result of which conductance reduces. The formation of micelles, thus, also reduces the osmotic pressure, thereby increasing the average molar mass of the hydrocarbon chain of the surfactant.)
of gas is absorbed per of a solid. A plot vs pressure at two different temperatures, and , shows
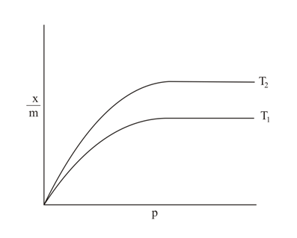
The plot of vs should be a straight line
