Cleansing Agents
Cleansing Agents: Overview
This topic explores the types of cleansing agents and includes chemical reactions showing their process of cleansing. It also covers the formation of cleansing agents.
Important Questions on Cleansing Agents
Label the hydrophilic and hydrophobic parts in the following compound.
Label the hydrophilic and hydrophobic parts in the following compound.
Explain the following term with suitable examples: Non-ionic detergents.
Explain the following term with suitable examples
Anionic detergents
Label the hydrophilic and hydrophobic parts in the following compound.
If water contains dissolved calcium hydrogen carbonate, out of soaps and synthetic detergents which one will you use for cleaning clothes?
Explain the cleansing action of soaps.
Can you use soaps and synthetic detergents to check the hardness of water?
Why do soaps not work in hard water ?
What are biodegradable and non-biodegradable detergents? Give one example of each.
Explain the following term with suitable examples: Cationic detergents.
How are synthetic detergents better than soaps?
Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group(s) present in the molecule. 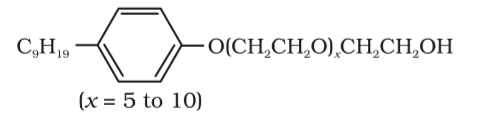
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structural formulae of these compounds are given below,
- Glyceryl palmitate.
- Glyceryl oleate.
