Covalent Bonding
Important Questions on Covalent Bonding
Draw the electron dot diagram of chemical bonds in methane and ethane .
Examples of some covalent compounds are given draw the chemical bonds of the compounds by using electron dot diagram.
Examine the illustration of the chemical bond formation in carbon tetrachloride molecule and answer the questions given below.
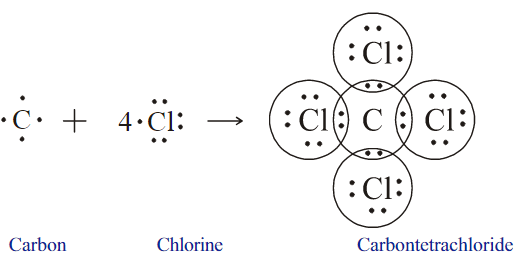
- How many pairs of electrons are shared by the carbon atom with each chlorine atom?
- What is the total number of electron pairs shared by the carbon atom with all the chlorine atoms?
- How can we represent the molecule using symbols?
Which type of chemical bond is possible in carbon tetrachloride molecule?
How many chlorine atoms have to combine with a carbon atom to complete its octet during the formation of carbon tetrachloride?
How many electrons are required for a chlorine atom to complete its octet?
How many electrons are required for a carbon atom to complete its octet?
Draw the electron dot diagram of carbon and chlorine.
Evaluate the illustration showing the formation of a chemical bond in hydrogen chloride molecule and answer the questions given below.
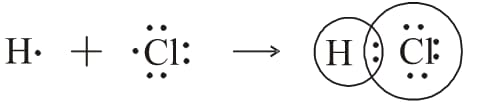
- How many electron pairs are shared?
- Represent chemical bond by using symbols?
Complete the table given below related to covalent bonding.
| Molecules of elements | Number of electron pairs shared | Chemical bond |
Examine the diagram illustrating the chemical bonding in the molecule of oxygen and nitrogen and answer the question given below.
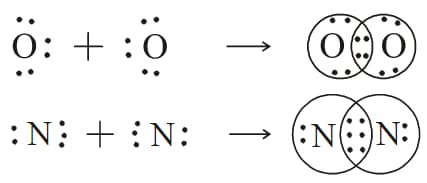
How many pairs of electrons are shared in these molecules?
Draw the electron dot diagram of a chlorine atom. Also, draw the electron dot diagram of the formation of chlorine molecule by combining two chlorine atoms? Determine the number of electron pairs shared.
The atomic number of chlorine is . Write its electronic configuration.
How many pairs of electrons are shared during the formation of a fluorine molecule?
What happens during the formation of fluorine molecule electron transfer or electron sharing?
How can the two fluorine atoms attain an octet arrangement?
How many electrons are required for one fluorine atom to attain the octet? Is ionic bonding possible in this molecule?

