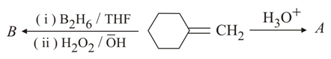Alcohols
Important Questions on Alcohols
Which one of the following compounds will be most dehydrated?
The final product in the sequence of reactions
What is in the following sequence of reactions?
Ethanol and dimethyl ether form a pair of functional isomers. The boiling point of ethanol is higher than that of dimethyl ether, due to the presence of:
During dehydration of alcohols to alkenes by heating with concentration the initiation step is:
An alkene, obtained by the dehydration of an alcohol , on ozonolysis gives two molecules of acetaldchyde for every molecule of an alkene. The alcohol is:
Vapour density of an organic compound is lt contains of carbon and of hydrogen. The compound gives iodoform test. The compound is:
Which type of carbocation is/are formed when
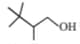 is treated with an acid?
is treated with an acid?
What is formed when a primary alcohol undergoes catalytic dehydrogenation?
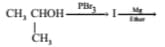
The reagent used for the preparation of higher ethers from halogenated ethers is
When ethylene glycol is heated with acidified potassium permanganate, the main organic compound obtained is
Osmium tetroxide is a reagent used for
Ethanol when reacts with gives , and . reacts with silver nitrite to form (major product) and . and , respectively are
An organic compound reacts with sodium metal and forms . On heating with concentrated , gives diethyl ether. and , respectively are
In the presence of alkali, reacts with to give
Which of the following is not true in case of reaction with heated copper at ?
The alcohol which does not give a stable compound on dehydration is
The reagent used for dehydration of an alcohol is