Gibbs Free Energy or Gibbs Function
Important Questions on Gibbs Free Energy or Gibbs Function
Given, and . The value of will be given by the slope of which line in the figure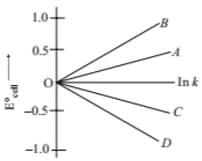
Which one of the following is correct?
A plot of against (abscissa) is expected to be a straight line with intercept on ordinate axis equal to
Which of the following conditions will apply to the conversion of ice into water?
For the precipitation reaction of ions with , which of the following statements is correct?
The densities of graphite and diamond at are and , respectively. If the standard free energy difference is equal to , the pressure at which graphite will be transformed into diamond at is
The most probable oxidation state of and will be
The factor of values is important in metallurgy. The values for the following reactions at are given as:
for the below reaction will be
Match List (Equations) with List (Types of process) and select the correct option.
| List- (Equations) |
List- (Types of process) |
||
|
Non-spontaneous |
|||
|
Equilibrium |
|||
| Spontaneous and endothermic | |||
| Spontaneous | |||
What is the free energy change for the conversion of mole of water into steam at . The heat of vaporization of water at is . The entropy change is .
In an irreversible process taking place at constant and and in which only the pressure-volume work is being done, the change in Gibbs free energy () and the change in entropy () satisfy the criteria
A certain reaction is non-spontaneous at . The entropy change during the reaction is . Is the reaction endothermic or exothermic? The minimum value of for the reaction is
What is the free energy change when of water at and pressure is converted into steam at and pressure?
Gibbs free energy for the reaction at equilibrium is
A spontaneous reaction is impossible if
Identify the correct statement for the change of Gibbs energy for a system () at constant temperature and pressure.
The enthalpy and entropy change for the reaction are and , respectively. The temperature at which the reaction will be in equilibrium is
Which of the following pairs of chemical reactions is certain to result in a spontaneous reaction?
The standard enthalpy and standard entropy changes for the oxidation of ammonia at are and , respectively. The standard Gibbs energy change for the same reaction at is
Standard Gibbs free energy change for isomerisation reaction cis- pentene trans- pentene is at . If more trans- pentene is added to the reaction vessel,

