Latent Heat or Hidden Heat
Important Questions on Latent Heat or Hidden Heat
The latent heat of vaporization of a substance is always:
The phenomenon of refreezing the water into ice on removing the increased pressure is called
Which of the following undergo sublimation?
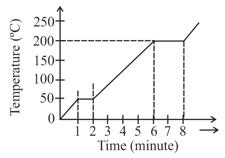
A student takes wax (specific heat and heats it till it boils. The graph between temperature and time is as follows. Heat supplied to the wax per minute and boiling point are respectively
A piece of ice (heat capacity and latent heat ) of mass grams is at at atmospheric pressure. It is given of heat so that the ice starts melting. Finally, when the ice-water mixture is in equilibrium, it is found that of ice has melted. Assuming there is no other heat exchange in the process, the value of is
In an energy recycling process, of steam at becomes water at which converts of ice at into water at . The ratio will be
of ice at is mixed with of water at . What will be the final temperature of the mixture?
Specific heat capacity of water is
Specific heat capacity of ice is
Latent heat of the water