D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 4: INTRODUCTORY EXERCISE 21.4
D. C. Pandey Physics Solutions for Exercise - D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 4: INTRODUCTORY EXERCISE 21.4
Attempt the practice questions on Chapter 5: Laws of Thermodynamics, Exercise 4: INTRODUCTORY EXERCISE 21.4 with hints and solutions to strengthen your understanding. Understanding Physics JEE Main & Advanced WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 4: INTRODUCTORY EXERCISE 21.4 with Hints & Solutions
In a heat engine, the temperature of the source and sink are, and . If the engine consumes per cycle, find (a) the efficiency of the engine, (b) work done per cycle, and (c) heat rejected to the sink per cycle.
A Carnot engine takes of heat from a reservoir at and gives it to a sink at . Find the work done by the engine.
The efficiency of a carnot cycle is . If on reducing the temperature of the sink by, the efficiency becomes , find the source and the sink temperatures between which the cycle is working.
Refrigerator works between and while refrigerator works between and , both removing heat equal to from the freezer. Which of the two is a better refrigerator?
A refrigerator has to transfer an average of heat per second from a temperature of to . Calculate the average power consumed assuming that no energy is lost in the process.
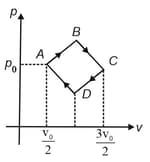
moles of a monoatomic gas is taken around in a cyclic process consisting of four processes along as shown. All the lines on the diagram have a slope of magnitude . The pressure at and is and the volumes at and are and , respectively. Calculate the percentage efficiency of the cycle.