David Sang and Graham Jones Solutions for Chapter: Quantum Physics, Exercise 7: Questions
David Sang Physics Solutions for Exercise - David Sang and Graham Jones Solutions for Chapter: Quantum Physics, Exercise 7: Questions
Attempt the practice questions on Chapter 28: Quantum Physics, Exercise 7: Questions with hints and solutions to strengthen your understanding. Physics for Cambridge International AS & A Level Coursebook 3rd Edition Digital Access solutions are prepared by Experienced Embibe Experts.
Questions from David Sang and Graham Jones Solutions for Chapter: Quantum Physics, Exercise 7: Questions with Hints & Solutions
Figure shows part of the energy level diagram for the electrons in an imaginary atom. The arrows represent three transitions between the energy levels. For each of these transitions, calculate the energy of the photon.
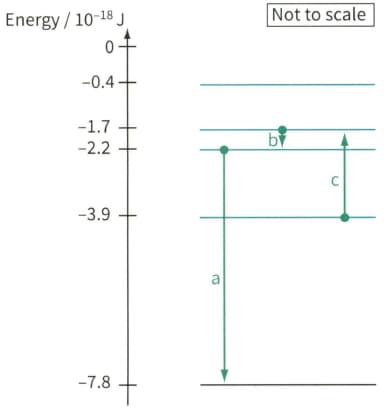
Figure, shows part of the energy level diagram for the electrons in an imaginary atom. The arrows represent three transitions between the energy levels. For each of these transitions:
Electron energy level diagram, showing three electron transitions a, b and c.
Calculate the frequency and wavelength of the electromagnetic radiation (emitted or absorbed)
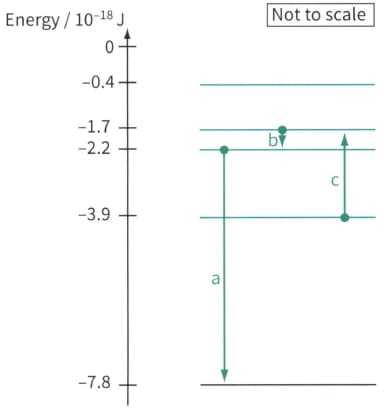
Figure, shows part of the energy level diagram for the electrons in an imaginary atom. The arrows represent three transitions between the energy levels. For each of these transitions:
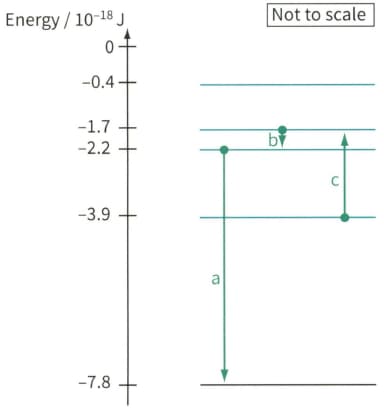
Electron energy level diagram, showing three electron transitions a, b and c.
State whether the transition contributes to an emission line in the spectrum or an absorption line in the spectrum.
Figure 28.20 shows another energy level diagram. In this case, energy is given in electronvolts (eV). The list shows the energies of some photons: . State and explain which of these photons will be absorbed by the electrons.
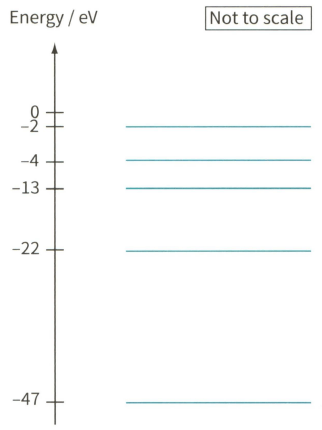
An energy level diagram.
The line spectrum for a particular type of atom is found to include the following wavelengths: . Calculate the corresponding photon energies in eV.
The line spectrum for a particular type of atom is found to include the following wavelengths: 83 nm 50 nm 25 nm.
(b) Sketch the energy levels that could give rise to these photons. On the diagram, indicate the corresponding electron transitions responsible for these three spectral lines.
