Dr. S P Jauhar and R Jauhar Solutions for Chapter: Chemical Reactions and Equations, Exercise 2: TEST YOURSELF
Dr. S P Jauhar Chemistry Solutions for Exercise - Dr. S P Jauhar and R Jauhar Solutions for Chapter: Chemical Reactions and Equations, Exercise 2: TEST YOURSELF
Attempt the practice questions on Chapter 1: Chemical Reactions and Equations, Exercise 2: TEST YOURSELF with hints and solutions to strengthen your understanding. MODERN'S ABC+ OF SCIENCE CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Dr. S P Jauhar and R Jauhar Solutions for Chapter: Chemical Reactions and Equations, Exercise 2: TEST YOURSELF with Hints & Solutions
Consider the activity of heating ferrous sulphate crystals in a test tube.
Complete the sentence:
This is a _____ type reaction.
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
Rahul took some zinc granules in a test tube and added dilute to it. He observed that the colour of zinc granules changed to.
Carbon dioxide turns the lime water milky due to the formation of
Solid calcium oxide was taken in a container and water was added slowly to it.
State the two observations made in the experiment.
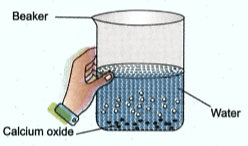
Solid calcium oxide was taken in a container and water was added slowly to it.
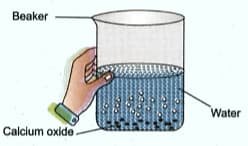
Write the name and the chemical formula of the product formed.
Crystals of copper sulphate are heated in a test tube for some time.
(a) What is the colour of copper sulphate crystals
(i) before heating and
(ii) After heating.
Crystals of copper sulphate are heated in a test tube for some time.
What is the source of liquid droplets seen on the inner upper side of the test tube during the heating process?
