Dr. S P Jauhar and R Jauhar Solutions for Chapter: Is Matter Around Us Pure?, Exercise 1: PRACTICE QUESTIONS
Dr. S P Jauhar Chemistry Solutions for Exercise - Dr. S P Jauhar and R Jauhar Solutions for Chapter: Is Matter Around Us Pure?, Exercise 1: PRACTICE QUESTIONS
Attempt the practice questions on Chapter 2: Is Matter Around Us Pure?, Exercise 1: PRACTICE QUESTIONS with hints and solutions to strengthen your understanding. Modern's abc+ of SCIENCE CHEMISTRY FOR Class IX solutions are prepared by Experienced Embibe Experts.
Questions from Dr. S P Jauhar and R Jauhar Solutions for Chapter: Is Matter Around Us Pure?, Exercise 1: PRACTICE QUESTIONS with Hints & Solutions
Sample 'A' contains iron filings and sulphur powder, Sample 'B' is iron sulphide. Sample 'A' and 'B' are taken on a watch glass and a bar magnet is rolled over both.
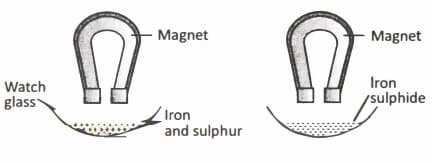
You will observe that:
In a china dish, of iron filings and of sulphur powder are mixed properly. Suggest a method to separate the individual constituents from its mixture.
A mixture of sand, ammonium chloride and sodium chloride is dissolved in water and filtered. The filtrate consists of:
To separate a mixture of iron filings, sulphur powder and iron sulphide, which sequence of methods should be used?
A mixture contains iodine, ammonium chloride and sand. Only iodine and ammonium chloride sublime. Only iodine dissolves in carbon tetrachloride.
How will you separate the three components? Sequence of steps will be:
A mixture of ammonium chloride and sodium chloride is heated in the apparatus as shown below:
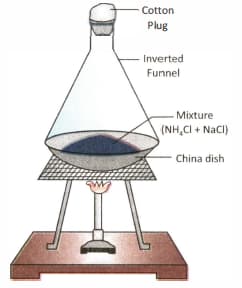
After the experiment, ammonium chloride will be obtained in the
Mixture of common salt and water can be separated completely by the process of:
A mixture containing iron filings and sulphur powder is spread on the white paper and a magnet is rolled in it. The particles which cling to the magnet are:
