Dr. S P Jauhar and R Jauhar Solutions for Chapter: Matter in Our Surroundings, Exercise 2: TEST YOURSELF
Dr. S P Jauhar Chemistry Solutions for Exercise - Dr. S P Jauhar and R Jauhar Solutions for Chapter: Matter in Our Surroundings, Exercise 2: TEST YOURSELF
Attempt the free practice questions on Chapter 1: Matter in Our Surroundings, Exercise 2: TEST YOURSELF with hints and solutions to strengthen your understanding. Modern's abc+ of SCIENCE CHEMISTRY FOR Class IX solutions are prepared by Experienced Embibe Experts.
Questions from Dr. S P Jauhar and R Jauhar Solutions for Chapter: Matter in Our Surroundings, Exercise 2: TEST YOURSELF with Hints & Solutions
When sugar dissolves in water, the level of water does not rise appreciably. Why?
For determining the melting point of ice, the thermometer should be kept:
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figure would correctly represent the result?
While determining the boiling point of water, the teacher suggested to add some pumice stone pieces to the hard glass test tube containing water. This was done to :
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
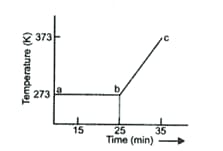
What does the curve ab represent?
(Choose from the following: Melting/ Boiling)
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
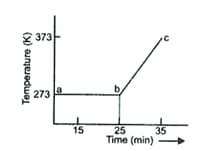
What happens after minutes?
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
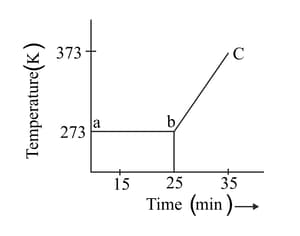
What does the curve bc represent?
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
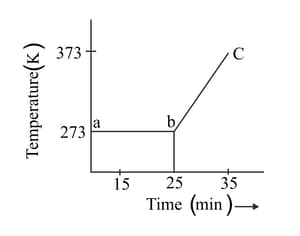
At what temperature and time ice melts?
