Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Main - 10th April 2015
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Main - 10th April 2015
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 1: JEE Main - 10th April 2015 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Main - 10th April 2015 with Hints & Solutions
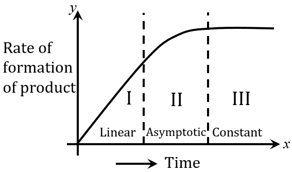
For certain chemical reaction , the rate of formation of product is plotted against the time as shown in the figure. The number of Correct statement/s from the following is _____
(A) Over all order of this reaction is one
(B) Order of this reaction can't be determined
(C) In region-I and III, the reaction is of first and zero order respectively
(D) In region-II, the reaction is of first order
(E) In region-II, the order of reaction is in the range of to .
For conversion of compound , the rate constant of the reaction was found to be . The order of the reaction is _____ .
If compound reacts with following first order kinetics with rate constant . The time taken by A (in seconds) to reduce from to will be _____ . (Nearest Integer)
An organic compound undergoes first order decomposition. If the time taken for the decomposition is , then the time required for decomposition will be is _____ s. (Nearest integer).
Given :
The rate constants of the above reaction at and are and respectively. The activation energy for the reaction is (Nearest integer)
(Given : In
The rate constant for a first order reaction is . The time required for the initial concentration of the reactant to reduce to its level is . (Nearest integer)
A and B are two substances undergoing radioactive decay in a container. The half life of A is and that of B is . If the initial concentration of B is times that of A and they both start decaying at the same time, how much time will it take for the concentration of both of them to be same? ________min.
The above reaction is of zero order. Half life of this reaction is . The time taken for the concentration of to reduce to one-fourth of its initial value is min . (Nearest integer)