Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: JEE Main - 1 February 2023 Shift 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: JEE Main - 1 February 2023 Shift 2
Attempt the free practice questions on Chapter 12: Classification of Elements and Periodicity in Properties, Exercise 1: JEE Main - 1 February 2023 Shift 2 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 1: JEE Main - 1 February 2023 Shift 2 with Hints & Solutions
The first ionization enthalpies of and follow the order
The first ionization enthalpy of and , respectively, are: and . The first ionization enthalpy of is
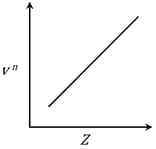
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
Which of the following represents the correct order of metallic character of the given elements?
Total number of acidic oxides among and is _____ .
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below:
The correct increasing order of the ionic radii is
For electron gain enthalpies of the elements denoted as , the incorrect option is :