Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: CG PET 2018
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: CG PET 2018
Attempt the practice questions on Chapter 7: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: CG PET 2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: CG PET 2018 with Hints & Solutions
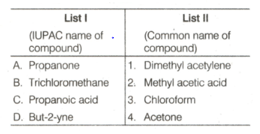
Match List-I with List-II and select the correct answer using the codes given below:
Which of the following statements are correct?
1. butanediol gives at STP when reacts with
2. Optically active iodobutane reacts with to form a racemic mixture.
3. methyl butane is optically active.
4. shows isomer.
Which of the following compound does not show geometircal isomerism?
The strongest acid amongst the following compounds is
Fusion mixture is
mole of a carbohydrate of empirical formula contains of hydrogen. The molecular formula of the carbohydrate is
of an organic compound contains of carbon, of hydrogen and the rest oxygen. The empirical formula of the compound is