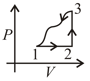First Law of Thermodynamics
Important Questions on First Law of Thermodynamics
A tyre pumped to a pressure of atmosphere, suddenly bursts. If the temperature of air before expansion is , then the final temperature of air is use
In an isothermal process if heat is supplied to an ideal gas, then
In an adiabatic process the volume is decreased by If , then the pressure is increased by
An insulated rectangular container carrying gas is filled with a movable piston. The dimension of the piston is The density of the material of which the piston is made is . If the gas expands, lifting the piston by, what is the change in the internal energy of the gas?
[Take ]

A gas undergoes a thermodynamic cycle as shown in pressure-volume diagram below. The heat transferred to the gas is and along path and respectively. The internal energy is changed by along path The work done by gas along path is