Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET Medical 2018 (22-Apr Shift-1)
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET Medical 2018 (22-Apr Shift-1)
Attempt the practice questions on Chapter 17: Thermodynamics, Exercise 1: AP EAPCET Medical 2018 (22-Apr Shift-1) with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET Medical 2018 (22-Apr Shift-1) with Hints & Solutions
If system is in thermal equilibrium with and is separately in thermal equilibrium with then and are in thermal equilibrium. From which thermodynamics law, does this follow?
Match the following: (where is gas constant)
| Column I | Column II | ||
| (a) | Molar specific heat of helium gas at constant volume | (i) | |
| (b) | Molar specific heat of oxygen at constant volume | (ii) | |
| (c) | Molar specific heat of carbon dioxide at constant volume | (iii) | |
| (d) | Molar specific heat of hydrogen at constant pressure | (iv) |
When the state of a gas is adiabatically changed from an equilibrium state to another equilibrium state the amount of work done on the system is If the gas is taken from state to via a process in which the net heat absorbed by the system is the net work done by the system in joule
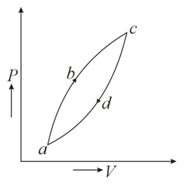
Figure below shows a cyclic process . If be the heat supplied to the system. be the change in internal energy and be the work done by the system, then which of the following relation is correct ?
What amount of heat is to be supplied to of Nitrogen at room temperature, to rise its temperature by at a constant pressure? and molecular weight of nitrogen is
Match the following?
| Column - I | Column - II | ||
| (a) | Isothermal process | (i) | No heat exchange |
| (b) | Adiabatic process | (ii) | Constant temperature |
| (c) | Isochoric process | (iii) | Constant pressure |
| (d) | Isobaric process | (iv) | Constant volume |
By leaving the door of a small standard domestic refrigerator open, it is not possible to cool a room, because it violates the ______
An ideal gas heat engine operates in Camot cycle between and It absorbs at the higher temperature. the amount of heat converted into work is equal to:
