Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: CG PET 2019
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: CG PET 2019
Attempt the practice questions on Chapter 14: Thermodynamics, Exercise 1: CG PET 2019 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: CG PET 2019 with Hints & Solutions
An insulated box containing a diatomic gas of molar mass is moving with a velocity . The box is suddenly stopped. The resulting change in temperature is (Where, is the gas constant)
For a gas if then atomicity, specific heat capacity at constant pressure and specific heat capacity at constant volume of the gas are respectively
mole of gas with is mixed with mole of gas with , then the value of for the resulting mixture is
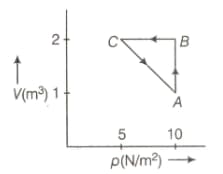
An ideal gas is taken through the cycle , as shown in the figure below. If the net heat supplied to the gas is , then the work done by the gas in the process is
The volume of a gas is reduced adiabatically to of its volume at . If , then the new temperature will be
A diatomic ideal gas is compressed adiabatically to of its initial volume. If the initial temperature of the gas is (in kelvin) and the final temperature is , the value of is
Which of the following is not true about the process?
For which of the following processes, entropy change is zero?
