Acid-Base Indicators
Acid-Base Indicators: Overview
This topic talks about indicators of acids and bases. It also outlines the natural as well as the synthetic indicators which help identify acids and bases. The colour changes in these indicators show us the presence of acids and bases.
Important Questions on Acid-Base Indicators
A student was given a solution to find its pH. His teacher declared his recorded pH as wrong. Student explained to his teacher, all the steps done by him while finding pH of sample. Mark the step taken by student in which he committed mistake.
The products of reaction between zinc and sodium hydroxide solution are :
A student was provided with four samples of solutions as shown in figures (I), (Il), (Ill), and (IV). He determined pH value of each solution by using pH paper. The correct sequence of colour change of pH paper observed by the student will be:
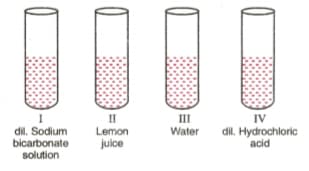
A student added a drop of universal indicator to 1 ml of the given solution and found that a green colour is produced. pH value of the solution will be in the range of :
When a few drops of universal indicator were added to a dilute solution of , it is observed that the colour of the solution changes from:
If is added to distilled water, the pH of new solution will be :
The pH value of a sample of hydrochloric acid is 2. pH value of this sample when diluted by adding water will be:
On adding methyl orange to a solution 'A' it imparts a pink colour and on adding it to solution 'B' a yellow colour is obtained, A and B solutions are respectively :
Some drops of acetic acid are poured over a pH paper. The colour produced on the paper will be
Identify the colour change in pH paper when a drop of a sample; which has pH 14 in standard pH colour chart is placed on it :
The colour of pH strip turned red when it was dipped in a sample. The sample could be :
On putting a few drops of an unknown liquid on the pH strip, the colour of the pH strip is changed to violet. This liquid taken is likely to be :
The correct method of finding pH of the solution is to:
On putting a few drops of a liquid on a pH strip, the colour of pH strip changed to green. The liquid is most probably.
A student was given three samples containing hydrochloric acid, sodium bicarbonate solution and water in test-tubes l, Il and III respectively. On dipping a pH paper in them, he observed that the colour turned orange in I, blue in Il, and green in Ill. If arranged in increasing order of their pH it would be :
of a solution is , Its is:
When a few drops of universal indicator were added to a dilute solution of HCI, it is observed that the colour of the solution changes from
Which of the following can be used as an acid-base indicator by a visually impaired student?
On dilution pH of an acidic solution:
Among the following, lowest value of pH is shown by:
