Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise
Attempt the free practice questions on Chapter 12: Thermodynamics, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Achieve Indian Airforce Agniveer Vayu Physics Practice Book solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise with Hints & Solutions
"Two systems in thermal equilibrium with a third system separately are in thermal equilibrium with each other." This statement refers to which law of thermodynamics?
Pressure , volume and temperature of a certain material are related by where is constant. Work done by the material when temperature changes from to and pressure remains constant is
For a gas where is the universal gas constant and is the molar specific heat at constant volume. The gas is made up of molecules which are
Find the percentage decrease in the volume if the pressure of an ideal gas is increased by at constant temperature.
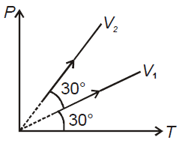
graph for same amount of an ideal gas is allowed to expand as shown in the figure below. Then which of the following is right?
You are riding on your bicycle with inflated tyres. Your friend asks for a lift and sits on the carrier behind you
Which type of molecular motion does not contribute towards internal energy:-
An ideal gas is reversibly around the cycle as shown on the (temperature) (entropy) diagram
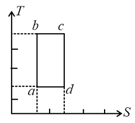
The most appropriate representation of the above cycle on a (internal energy) (volume) diagram is-
