Embibe Experts Solutions for Chapter: Equilibrium, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Equilibrium, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 5: Equilibrium, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Equilibrium, Exercise 4: Exercise-4 with Hints & Solutions
The following equilibrium exists in a saturated solution of
A change that will shift the equilibrium to the right is
A week acid, has a of . If of this acid is dissolving in of water, the percentage of acid dissociated at equilibrium is closed to
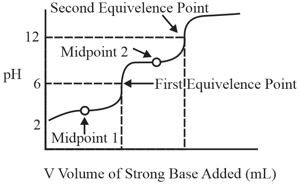
A solution of a substance is titrated against a strong base (or acid), volume of the strong base (or acid) is plotted against of the solution (as shown in the figure). The substance could be:
Select the correct graph(s) for the corresponding acid-base titration:
of a clear saturated solution of is added to of a clear saturated solution of . Then select the incorrect option(s). Given values for and are and respectively.
Calculate and in a solution saturated with respect to both
Given: .
A sample of was treated with of solution to give . The remaining solution contained per litre Then .
The melting points of most of the solid substances increases with an increase of pressure acting on them. However, ice melts at a temperature lower than its usual melting point, when the pressure increases. This is because:
