Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 4: Exercise-4
Attempt the practice questions on Chapter 25: Haloalkanes and Haloarenes, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 4: Exercise-4 with Hints & Solutions
The number of transition states in an unimolecular nucleophilic substitution () reaction is:
The sequence of steps involved in aromatic nucleophilic substitution involving a benzyne intermediate is:
The compound which undergoes hydrolysis on just warming with water and forms the corresponding hydroxyl derivative is:
chlorobenzene is mono-nitrated to .
nitrobenzene is mono-chlorinated to .
anisole is mono-nitrated to .
nitrochlorobenzene is mono-nitrated to .
Out of and , the compound that undergoes reaction with aqueous the fastest is:
The compound that undergoes solvolysis in aqueous ethanol most easily is:
The best reaction sequence to convert methylbromopropane into methylbromopentane is:
The compound that will not react with hot concentrated aqueous alkali at atmospheric pressure is:
Match List (Reaction) with List (Mechanism) and select the correct answer using the code given below the lists:
| List I | List II | ||
| P | 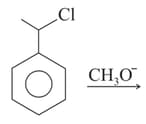 |
1 | |
| Q | 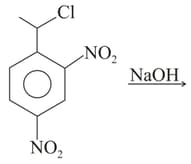 |
2 | |
| R | 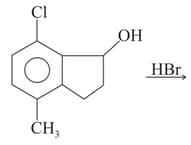 |
3 | |
| S | 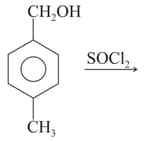 |
4 |
