Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 3: Exercise - 3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 3: Exercise - 3
Attempt the free practice questions on Chapter 33: Atomic Physics, Exercise 3: Exercise - 3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 3: Exercise - 3 with Hints & Solutions
If the mass of the particle is and , the energy of the particle in its ground state is closest to:
A diatomic molecule has moment of inertia . By Bohr’s quantization condition its rotational energy in the nth level ( is not allowed) is :
It is found that the excitation frequency from ground to the first excited state of rotation for the molecule is close to . Then the moment of inertia of molecule about its centre of mass is close to (Take )
Hydrogen Deuterium singly ionised Helium and doubly ionised lithium all have one electron around the nucleus. Consider an electron transition from If the wavelengths of emitted radiation are respectively then approximately which one of the following is correct?
As an electron makes a transition from an excited state to the ground state of a hydrogen - like atom/ion:
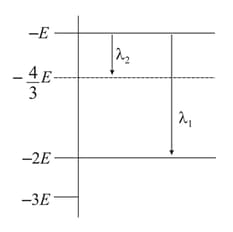
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths is given by :
If the series limit frequency of the Lyman series is then the series limit frequency of the Pfund series is:
An electron from various excited states of hydrogen atom emits radiation to come to the ground state. Let be the de-Broglie wavelength of the electron in the state and the ground state respectively. Let be the wavelength of the emitted photon in transition from the state to the ground state. For large
