Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise - 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise - 1
Attempt the free practice questions on Chapter 18: Kinetic Theory of Gases, Exercise 1: Exercise - 1 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise - 1 with Hints & Solutions
The mean speed of the molecules of a hydrogen sample equals the mean speed of the molecules of a helium sample. Calculate the ratio of the temperature of the hydrogen sample to the temperature of the helium sample.
of is kept in a closed container, initially at . The container is now heated. Neglecting the expansion of the container, calculate the temperature at which the internal energy is increased by .
For a gas sample with number of molecules, the function is given by, for and for . Where is a number of molecules in speed range to . The rms speed of the molecules is
and Are three closed vessels, at the same temperature And contain gases which obey the Maxwellian distribution of velocities. Vessel Contains only undefined and A mixture of equal quantities of And undefined. If the average speed of Molecules in vessel is , that of the undefined molecules in vessel is , the average speed of the Molecules in vessel is
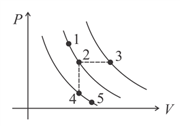
A certain gas is taken to the five states represented by dots in the graph. The plotted lines are isotherms. The order of the most probable speed of the molecules at these five states is,
The pressure of an ideal gas is written as, . Here, stands for,
The quantities which remain same for all ideal gases at the same temperature is/are,
One mole of an ideal gas undergoes a process in which, , where and are positive constants and is volume. The minimum pressure attainable is,
