Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise - 3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise - 3
Attempt the practice questions on Chapter 18: Kinetic Theory of Gases, Exercise 3: Exercise - 3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise - 3 with Hints & Solutions
A mixture of moles of helium gas (atomic mass) and mole of argon gas (atomic mass) is kept at in a container. The ratio of the rms speed is
Two non-reactive monoatomic ideal gases have their atomic masses in the ratio . The ratio of their partial pressure, when enclosed in a vessel kept at a constant temperature is . The ratio of their densities is
One kg of a diatomic gas is at a pressure of . The density of the gas is . What is the energy of the gas due to its thermal motion?
Three perfect gases at absolute temperature and are mixed. The masses of molecules are , and and the number of molecules are and , respectively. Assuming no loss of energy, the final temperature of the mixture is
A container with insulating walls is divided into two equal parts by a partition fitted with a valve. One part is filled with an ideal gas at a pressure and temperature whereas the other part is completely evacuated. If the valve is suddenly opened, then the pressure and temperature of the gas will be
The value of is undefined. Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as undefined. where is the volume of the gas.
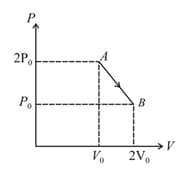
of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be
The temperature of an open room of volume increased from due to the sunshine. The atmospheric pressure in the room remains . If and are the number of molecules in the room before and after heating, then will be
