Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise-2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise-2
Attempt the practice questions on Chapter 33: Atomic Physics, Exercise 2: Exercise-2 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise-2 with Hints & Solutions
If Bohr's theory is applicable to then radius of this atom in Bohr's unit is
Figure shows the intensity-wavelength relations of -rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube in which the potential difference between the target and the filament is and the atomic number of the target material is These quantities are and for the other tube. Then,
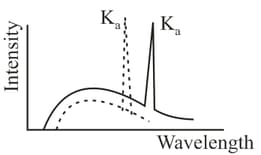
Wavelengths of lines of two elements are and , respectively. Number of elements between these elements in the sequence will be
For soft -rays the attenuation constant for aluminium is Then the percentage of -rays that will pass through an aluminium sheet of thickness will be
An -ray tube, when operated at tube voltage, records an anode current If the efficiency of the tube for production of -rays is then the heat produced per second in calories is nearly
Assertion: Neutrons penetrate matter more readily as compared to protons.
Reason: Neutrons are slightly more massive than protons.
Assertion : Bohr had to postulate that the electrons in stationary orbits around the nucleus do not radiate.
Reason : According to classical physics all moving electrons radiate.
Assertion: The hydrogen atom consists of only one electron, but its emission spectrum has many lines.
Reason: Only the Lyman series is found in the absorption spectrum of hydrogen atoms whereas in the emission spectrum all the series are found.
