Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise-1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise-1
Attempt the practice questions on Chapter 18: Kinetic Theory of Gases, Exercise 1: Exercise-1 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 1: Exercise-1 with Hints & Solutions
When the temperature of an ideal gas is increased from to its rms speed is changed from to The is:
Hydrogen gas is filled in a container of volume . Average translational kinetic energy of all its molecules is Pressure of hydrogen in cylinder is:
Root mean square speed of ideal hydrogen gas in closed chamber at is . Its pressure will be (density of hydrogen gas is )
A flask contains argon and chlorine in the ratio by mass. The temperature of the mixture is If atomic mass of argon and molecular mass of chlorine then the ratio of average kinetic energy per molecule of argon to chlorine gas is:
The internal energy of a mono-atomic gas is,
According to law of equal distribution of energy, the mean energy of a molecule per degree of freedom is:
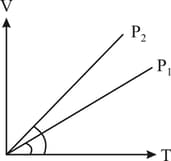
In the following diagram what is the relation between and
An electric fan is switched on in a closed room. The air in the room is___.
