Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Exercise-3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Exercise-3
Attempt the practice questions on Chapter 17: Thermodynamics, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Exercise-3 with Hints & Solutions
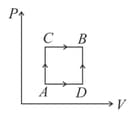
moles of a monoatomic gas is carried round the reversible rectangular cycle as shown in the diagram. The temperature at is . The thermodynamic efficiency of the cycle is approximately
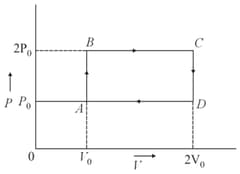
The net-work done on the gas in the cycle is:
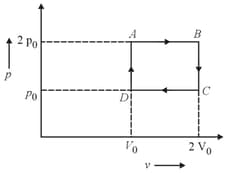
Helium gas goes through a cycle (consisting of two isochoric and isobaric lines) as shown in the figure. The efficiency of this cycle is nearly (assume the gas to be close to ideal gas)
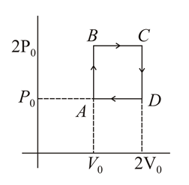
The shown diagram represents the thermodynamic cycle of an engine operating with an ideal mono atomic gas. The amount of heat extracted from the source in a single cycle is
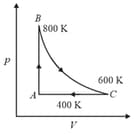
One mole of diatomic ideal gas undergoes a cyclic process as shown in the figure. The process is adiabatic. The temperature at and are , and , respectively. Choose the correct statement.
and are specific heats at constant pressure and constant volume, respectively. It is observed that for hydrogen gas, for nitrogen gas. The correct relation between and is
A gas can be taken from to via two different processes and . When path is used of heat flows into the system and of work is done by the system. If path is used work done by the system is . The heat flow into the system in path is
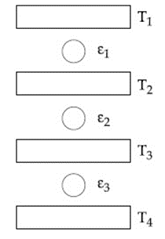
Three Carnot engines operate in series between a heat source at a temperature and a heat sink at temperature (see figure). There are two other reservoirs at temperature and as shown with The three engines are equally efficient if:
A rigid diatomic ideal gas undergoes an adiabatic process at room temperature. The relation between temperature and volume for this process is constant, then is:
