Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 3: EXERCISE-3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 3: EXERCISE-3
Attempt the free practice questions on Chapter 24: Hydrocarbons, Exercise 3: EXERCISE-3 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 3: EXERCISE-3 with Hints & Solutions
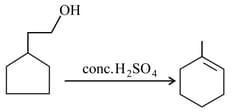
How many -shifts of carbocation intermediate are involved during the course of following reaction:
Number of possible mono chloro derivative products of -methyl butane is?
Number of compound which can show faster rate of than 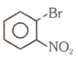
(1)
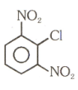
(2)

(3)
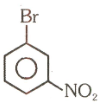
(4)
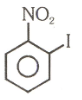
(5)
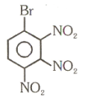
(6)
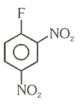
(7)
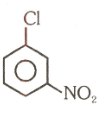
Number of compound which cannot involve in Fridal craft reaction.
(1)
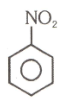
(2)
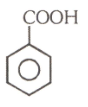
(3)
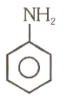
(4)
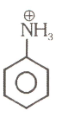
(5)
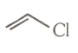
(6)

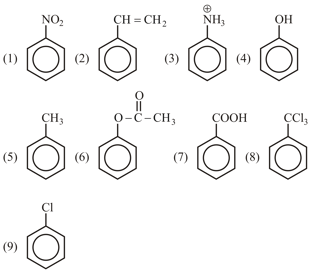
Among the following, the number of ortho para directing with activating group.
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
Among the following the number of reagents can be used to generate in nitration of benzene rings..
(1) conc. conc.
(2)
(3) Solid
(4) conc. conc.
(5) conc.
(6)
Number of compounds that gives a tribromo derivative on treatment with bromine water.
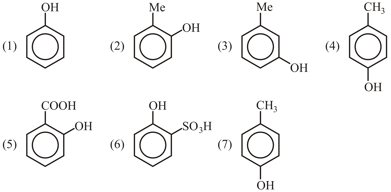
Electrophilic attack of at meta position is observed in