Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: EXERCISE-2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: EXERCISE-2
Attempt the free practice questions on Chapter 4: Thermodynamics, Exercise 2: EXERCISE-2 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: EXERCISE-2 with Hints & Solutions
For the which of the following reactions. will the maximum:
The entropy change when two moles of ideal monoatomic gas is heated from to reversibly and isochorically?
When two equal sized pieces of the same metal at different temperatures (hot piece) and (cold piece) are brought into contact into thermal contact and isolated from its surrounding. The total change in entropy of system is given by?
What can be concluded about the value of and from this graph?
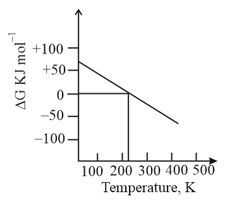
For which of the following processes, total entropy of universe increases.
mole of an ideal gas at is subjected to expand reversibly ten times of its initial volume. The change in entropy due to expansion is:
Chemist's seemingly insatiable need to measure heat has led to a broad spectrum of measurement methods. A special calorimeter is the ice calorimeter, in which the heat released in an exothermic reaction is trapped in an ice-water mixture at , causing ice to melt which result in contraction of volume. A student employs this method to determine the heat of combustion of methanol. He finds that when a sample of methanol weighing is burnt in excess oxygen in an ice calorimeter at constant volume and , according to the reaction,
the volume of ice and water surrounding the sample decreases by .
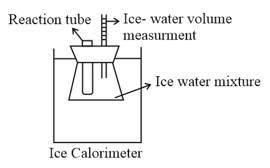
Given: specific volume of ice , specific volume of , of ice
Select the correct option(s)
Aluminium and is used as fuel to produce energy according to given reaction.
Given:
Density of aluminium
Density of
Select the correct statement(s).
