Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise-1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise-1
Attempt the practice questions on Chapter 17: Thermodynamics, Exercise 1: Exercise-1 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise-1 with Hints & Solutions
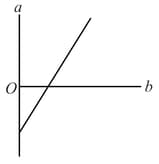
The expansion of unit mass of a perfect gas at constant pressure is shown in the diagram. Here:
Air is filled at in a vessel of open mouth. The vessel is heated to a temperature so that part of air escapes. The value of is:
One mole of an ideal gas undergoes a process , here and are constants. Change in temperature of the gas when the volume is changed from to is:
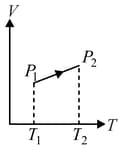
From the following diagram we can conclude:
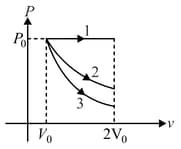
A gas is expanded from volume to under three different processes. Process is isobaric process, Process is isothermal and Process is adiabatic. Let and , be the change in internal energy of the gas is these processes. Then:
The internal energy of a gas in an adiabatic process is given by , find
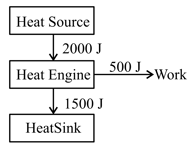
What would be the efficiency of the heat engine diagramed as shown below?
An ideal Carnot heat engine with an efficiency of . It absorbs heat from a hot reservoir at . The temperature of the cold reservoir is
