Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise-2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise-2
Attempt the free practice questions on Chapter 17: Thermodynamics, Exercise 2: Exercise-2 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise-2 with Hints & Solutions
One mole of an ideal monatomic gas is taken from to along the path The temperature of the gas at is For the process
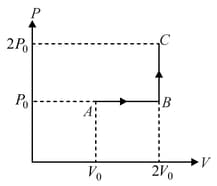
The specific heats of a gas are . (Take )
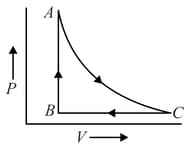
One mole of monoatomic ideal gas undergoes a cyclic process as shown in figure. Process is adiabatic. The temperatures at and are and respectively:-
A gas expands such that its initial and final temperatures are equal. Also, the process followed by the gas traces a straight line on the diagram :-
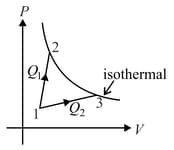
A gas takes part in two processes in which it is heated from the same initial state to the same final temperature. The processes are shown on the diagram by the straight line and and are the, points on the same isothermal curve. and are the heat transfer along the two processes. Then :-
Three identical adiabatic containers have helium, neon and oxygen gases at the same pressure. The gases are compressed to half their original volume. Then:-
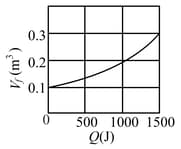
Suppose mole of an ideal gas undergoes an isothermal expansion as energy is added to it as heat . Graph shows the final volume versus . The temperature of the gas is :- (use and )
A sample of gas follows process represented by constant. Bulk modulus for this process is , then which of the following graph is correct?
