Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 3: EXERCISE-II (Previous Year Questions)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 3: EXERCISE-II (Previous Year Questions)
Attempt the practice questions on Chapter 12: Classification of Elements and Periodicity in Properties, Exercise 3: EXERCISE-II (Previous Year Questions) with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Classification of Elements and Periodicity in Properties, Exercise 3: EXERCISE-II (Previous Year Questions) with Hints & Solutions
The atomic number is . in the same group the atomic number of elements placed above and below will be:
In the general electronic configuration - if value of the configuration will be of -
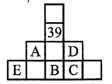
On the basis of the given part of the periodic table, an incorrect statement is
If total elements are present in the periodic table then how many of them contain in the subshell:
The internuclear distance in and molecules are and , respectively. The bond length of maybe.
These are elements and . Their atomic number are , respectively. If and and the electronic configuration of an element is the correct order of atomic radius is:
If are ionisation energies, the which of the following order is not correct -
If electron affinity of an element is than ionisation potential of this element:
