Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: BEGINNER'S BOX
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: BEGINNER'S BOX
Attempt the practice questions on Chapter 5: Equilibrium, Exercise 1: BEGINNER'S BOX with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: BEGINNER'S BOX with Hints & Solutions
For the equilibrium
What is the temperature at which
For the reaction
if the initial concentration of and moles of is consumed at equilibrium, the correct expression of is.
Which of the following is correct regarding the gas-solution equilibrium ?
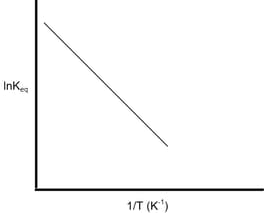
According to this graph, the reaction will be:
For the reaction , if , where the symbols have usual meaning then the value of is: (assuming ideality)
is converted into according to the reaction . Value of at this point is :-
Which of the following salts undergoes anionic hydrolysis?
reaction is said to be in equilibrium, when:
