Embibe Experts Solutions for Chapter: Atomic Structure, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Atomic Structure, Exercise 1: Exercise 1
Attempt the free practice questions on Chapter 2: Atomic Structure, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course BITSAT solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Atomic Structure, Exercise 1: Exercise 1 with Hints & Solutions
The graph that depicts Einstein's photoelectric effect for a monochromatic source of frequency above the threshold frequency is
The correct representation of wavelength intensity relationship of an ideal blackbody radiation at two different temperatures and is"
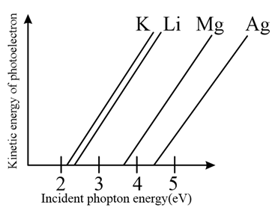
The photoelectric behaviour of and metals is shown in the plot below. If light of wavelength is incident on each of these metals, which of them will emit photoelectrons? [Planck's constant and ]
A object is moving with a velocity of . The de-Broglie wavelength(in ) of the object is[Planck's constant, ]
For a orbital, the number of radial and angular nodes, respectively, are:
Maximum number of electrons that can be accommodated in the subshell with azimuthal quantum number , is:
The maximum number of electrons that can be filled in the shell with the principal quantum number is:
The electronic configuration which obeys Hund's rule for the ground state of carbon atom is:
