Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 13: Solid State, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1 with Hints & Solutions
The interestial hydrides having the same lattice as the parent metal
Which of the following species have integral bond order, gerade type and paramagnetic nature.
Which mineral can have the silicates skeleton as shown below?
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
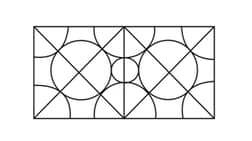
[Hint: Calculate the radius of the smallest circle in the figure.]
A metal cystallises into two cubic phases, f.c.c and b.c.c. whose unit-cell lengths are and . Calculate the ratio of densities of f.c.c. and b.c.c.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
Potassium metal crystallizes in a face-centred arrangement of atoms where the edge of the unit cell is . Determine the shortest separation of any two potassium nuclei in pm is.
Sodium metal crystallizes in a body-centred cubic lattice with the cell edge . What is the radius of the sodium atom in pm? (Round off your answer to the nearest integer value.)
A crystal of sodium Hydride has fcc unit cell of ions with ions at the body centres and edge centres. The No. of ions that touches each ion is:
