Embibe Experts Solutions for Chapter: Solid State, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solid State, Exercise 2: Level 2
Attempt the practice questions on Chapter 21: Solid State, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solid State, Exercise 2: Level 2 with Hints & Solutions
Lithium crystallizes in a body centred cubic lattice. How many next-nearest neighbors does each have?
Which of the following shaded planes in lattice contains the arrangement of atoms as shown by the circles_____

Consider the radii Choose the correct option from the following. (Use radius ratio rules)
The density of crystalline is . The volume, effectively occupied by a single ion-pair in the crystal is:
The number of atoms in of an f.c.c. crystal, with density and cell edge equal to , is equal to
An element (atomic mass ) having b.c.c. structure has unit cell edge . The density in of the element is
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
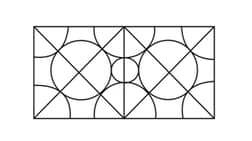
[Hint: Calculate the radius of the smallest circle in the figure.]
A face-centred cubic solid of an element (atomic mass ) has a cube edge of Calculate its density.
