Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 2: Level 2
Attempt the practice questions on Chapter 26: Surface Chemistry, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 2: Level 2 with Hints & Solutions
The pressure of the gas was found to decrease from to of Hg. When of the sample of activated charcoal was kept in a flask of one litre capacity maintained at . If the density of charcoal was at . The volume of gas adsorbed per of charcoal at of is:
Graph between and is a straight line inclined at an angle of When pressure is and the amount of solute adsorbed per of the adsorbent will be:
The adsorption isotherm for a gas is given by the relation , where is moles of gas adsorbed per gram of the adsorbent, is the pressure of the gas, and and are constants. Then, :
During the process of digestion, the proteins present in food materials are hydrolysed to amino acids. The two enzymes involved in the process:
ProteinsPolypeptidesAmino acids, are respectively
The enzymes having two sites are
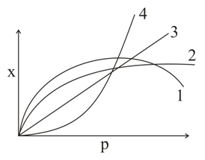
The curve that best describes the adsorption of a gas on of a solid substrate as a function of pressure at a fixed temperature:
Which of the following ions is the most effective in the coagulation of an arsenious sulphide solution?
Finely divided catalysts have greater surface area and have greater catalytic activity than the compact solid. If a total surface area of is required for adsorption in a catalysed gaseous reaction, how many splits should be made to a cube of exactly in length to achieve required surface area. (Given: One split of a cube gives eight cubes of same size)?
