Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1
Attempt the free practice questions on Chapter 11: Solid State, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course MHT-CET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solid State, Exercise 1: Exercise 1 with Hints & Solutions
A solid is hard and brittle. It is an insulator in solid state but conducts electricity in molten state. The solid is a
In metallic solids, the number of atoms for the face centred and the body-centred cubic unit cells are, respectively.
In a cubic close packed structure, the fractional contributions of an atom at the corner and at the face in the unit cell are, respectively:
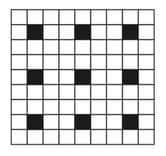
A two-dimensional solid pattern formed by two different atoms and is shown below. The black and white squares represent atoms and , respectively. The simplest formula for the compound based on the unit cell from the pattern is
In the cubic structure of a compound which is made from and , atoms are at the corners of the cube and atoms are at the face centers of the cube. The molecular formula of compound is
A metal with an atomic radius of crystallizes in the face centred cubic structure. The volume of the unit cell in is
The packing efficiency of the face centred cubic , body centred cubic and simple/primitive cubic lattices follows the order
Schottky defect in a crystal arises due to
