Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: Level 2
Attempt the practice questions on Chapter 10: Chemical Kinetics, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: Level 2 with Hints & Solutions
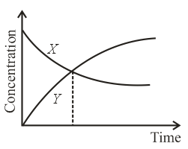
The accompanying figure depicts the change in concentration of species and for the elementary reaction as a function of time. The point of intersection of two curves represents:
Consider the reaction product. When concentration of alone was doubled the half-life did not change. When concentration of alone was doubled the rate increased by two times. The unit of rate constant for this reaction is:
If for the reaction then in will be:
for a given hypothetical reaction. If rate expression is then the ratio of and will be:
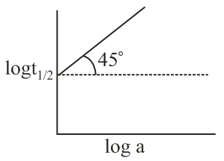
What will be the order of reaction for a chemical change having the graph between vs as shown below? where, initial concentration of reactant;
For an elementary reaction , if the volume of vessel is quickly reduced to half of its original volume, then what will happen to the rate of reaction?
For the zero-order reaction the initial concentration of is . If the concentration of after minutes, then, its half-life and completion time are respectively
For an ideal gaseous reaction, the rate is expressed in terms of instead of (where is concentration and is the number of moles). What is the relation among these expressions, if and are constant?
