Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 3: Level 3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 3: Level 3
Attempt the practice questions on Chapter 6: Redox Reactions, Exercise 3: Level 3 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 3: Level 3 with Hints & Solutions
reacts with water and alkali to give:
Hydrogen peroxide in aqueous solution decomposes on warming to give oxygen according to the equation: under conditions where mole of gas occupies . of solution of produces of . Thus, is:
The oxidation states of sulphur in the anions and follow the order:
Match List (Compounds) with List (Oxidation states of Nitrogen) and select the answer using the codes given below the lists:
| List | List | ||
| a | 1 | ||
| b | 2 | ||
| c | 3 | ||
| d | 4 |
The resonating structures of the cyanate ion are 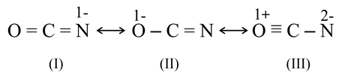 . The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
. The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
is oxidised by in the presence of an acid : . What are the whole number values of in that order?
For the redox-reaction ,
the correct whole number stoichiometric coefficients of and , respectively are
is oxidised by in presence of acid:
What are the whole number values of in that order:
