Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 3: Level 3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 3: Level 3
Attempt the practice questions on Chapter 2: Structure of Atom, Exercise 3: Level 3 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 3: Level 3 with Hints & Solutions
In Bohr series of lines of hydrogen spectrum, the third line from the red-end corresponds to which one of the following inter-orbit jumps of the electron for Bohr orbits in an atom of hydrogen?
A certain metal when irradiated by light emits photoelectrons with twice kinetic energy as did photoelectrons when the same metal is irradiated by light . The of metal is
The frequency of radiation emitted when electron falls from to in a hydrogen atom will be
(Given: Ionization energy of and )
A metal is irradiated with a light of wavelength . Given that the work function of the metal is , the de-Broglie wavelength of the ejected electron is close to
A bulb of is producing a light of wavelength with of efficiency, then the number of photons emitted by the bulb in seconds are:
A plot of the kinetic energy of ejected electrons as a function of the frequency of incident radiation for four alkali metals is shown below:
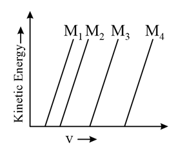
The alkali metals and are, respectively:
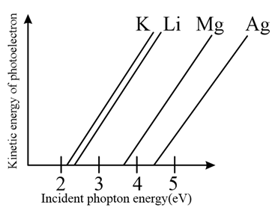
The photoelectric behaviour of and metals is shown in the plot below. If light of wavelength is incident on each of these metals, which of them will emit photoelectrons? [Planck's constant and ]
The correct representation of wavelength intensity relationship of an ideal blackbody radiation at two different temperatures and is"
