Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 1: AMU-AT (B.Tech.) 2018
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 1: AMU-AT (B.Tech.) 2018
Attempt the free practice questions on Chapter 25: Aldehydes, Ketones and Carboxylic Acids, Exercise 1: AMU-AT (B.Tech.) 2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 1: AMU-AT (B.Tech.) 2018 with Hints & Solutions
Cinnamic acid is formed when condenses with in presence of
Aldehydes and ketones can be reduced to hydrocarbon by using
On heating an aldehyde with Fehling's reagent, a reddish brown precipitate is obtained due to the formation of
A compound forms a phenylhydrazone and gives negative Tollen's test and a positive Iodoform reaction. It also gives -pentane on reduction. The compound is
A -hydroxy carbonyl compound is obtained by the action of on
Name the end product in the following series of reactions ections
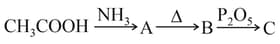
The most reactive to nucleophilic attack at the carbonyl group is
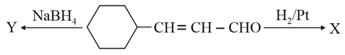
What are and
