Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: JEE Main - 10th April 2015
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: JEE Main - 10th April 2015
Attempt the free practice questions on Chapter 13: Chemical Bonding and Molecular Structure, Exercise 1: JEE Main - 10th April 2015 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: JEE Main - 10th April 2015 with Hints & Solutions
The magnetic behaviour of , and , respectively, are
According to theory the bond orders for , and respectively, are
Match List I with List II
|
List I (molecules/ions) |
List II (No. of lone pairs of |
||
| (A) | I. | Three | |
| (B) | II. | One | |
| (C) | III. | Two | |
| (D) | IV. | Zero | |
Choose the correct answer from the options given below:
For molecule consider the following:
(A) Number of lone pairs on oxygen is .
(B) angle is less than .
(C) Oxidation state of is .
(D) Molecule is bent '' shaped.
(E) Molecular geometry is linear.
Correct options are:
Match List I with List II
| List I | List II |
| A. | I.See-saw |
| B. | II. Square planar |
| C. | III. Bent shaped |
| D. | IV. Tetrahedral |
Choose the correct answer from the options given below :
Amongst the following, the number of species having the linear shape is
and
Resonance in carbonate ion is
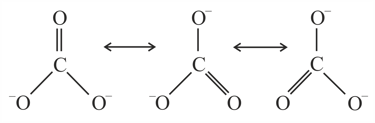
Which of the following is true?
bond length in is than the bond length in . The bond length in is than that of the bond in . Choose the correct option for and from the given below.
