Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: WBJEE 2019
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: WBJEE 2019
Attempt the practice questions on Chapter 4: Chemical Bonding and Molecular Structure, Exercise 1: WBJEE 2019 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: WBJEE 2019 with Hints & Solutions
The melting points of (i) (ii) and (iii) follows the order
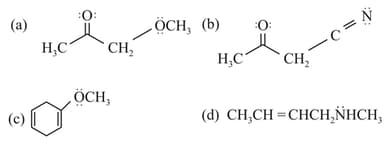
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
The angle in ammonia is while the angle in phosphine is Relative to phosphine, the -character of the lone-pair on ammonia is expected to be
Which of the following has the strongest H-bond ?
Bond order of and are respectively
A homonuclear diatomic gas molecule shows -electron magnetic moment. The one-electron and two-electron reduced species obtained from above gas molecule can act as both oxidising and reducing agents. When the gas molecule is one-electron oxidised the bond length decreases compared to the neutral molecule. The gas molecule is
Which of these species will have non-zero magnetic moment?
Which statements are correct for the peroxide ion ?
