Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: CG PET 2018
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: CG PET 2018
Attempt the practice questions on Chapter 12: Chemical Kinetics, Exercise 1: CG PET 2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: CG PET 2018 with Hints & Solutions
Graph plotted between versus log concentration is a straight line. What conclusion can you draw from the graph?
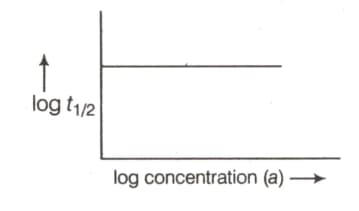
order of reaction
Half-life period for a first order reaction is minutes. How much time is required to change the concentration of the reactants to ?
The conversion of cis--dichloroethene to trans--dichloroethene is an example of
Consider the following reaction
The rate can be expressed as
If , what is the effect on rate constant of a reaction for rise in temperature?
It will increase
For gaseous reaction, the following data is given
The temperature at which is
The Arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature and may be expressed as . If the unit of is , the unit of A will be
