Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: NEET - 12 September 2021
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: NEET - 12 September 2021
Attempt the free practice questions on Chapter 17: Chemical Kinetics, Exercise 1: NEET - 12 September 2021 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: NEET - 12 September 2021 with Hints & Solutions
The given graph is a representation of kinetics of a reaction.
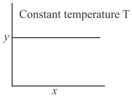
The and axes for zero and first order reactions, respectively are
For a first order reaction Products, initial concentration of is , which becomes after minutes. Rate constant for the reaction in is
For a reaction enthalpy of reaction is and enthalpy of activation is The correct potential energy profile for the reaction is shown in the option.
The slope of Arrhenius Plot of first order reaction is The value of of the reaction is. Choose the correct option for your answer.
[Given ]
The half-life for a zero order reaction having initial concentration of reactant is . The rate constant (in ) for the reaction is
In collision theory of chemical reaction, represents
The rate constant for a first order reaction is . The time required to reduce of the reactant to is :
An increase in the concentration of the reactants of a reaction leads to change in
