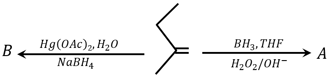Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: JEE Main - 15th April 2018
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: JEE Main - 15th April 2018
Attempt the free practice questions on Chapter 23: Hydrocarbons, Exercise 1: JEE Main - 15th April 2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: JEE Main - 15th April 2018 with Hints & Solutions
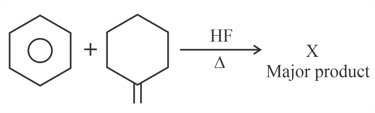
Find out the major products from the following reactions.
of a hydrocarbon (M.F. ) takes up of the gas measured at and of . Ozonolysis of the same hydrocarbon yields
The number of double bond/s present in the hydrocarbon is
The one giving maximum number of isomeric alkenes on dehydrohalogenation reaction is (excluding rearrangement)
The major products ‘’ and ‘’, respectively, are
Choose the correct set of reagents for the following conversion
trans
A hydrocarbon 'X' with formula uses two moles of on catalystic hydrogenation of its one mole. On ozonolysis, 'X' yields two moles of methane dicarbaldehyde. The hydrocarbon 'X' is :
But--yne is reacted separately with one mole of Hydrogen as shown below:
Identify the incorrect statements from the options given below:
A. A is more soluble than .
B. The boiling point & melting point of are higher and lower than respectively.
C. A is more polar than because dipole moment of is zero.
D. adds easily to than .
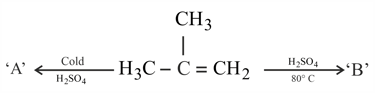
‘’ is: