Embibe Experts Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Kerala Board-2018
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Kerala Board-2018
Attempt the free practice questions on Chapter 3: Reactivity Series and Electrochemistry, Exercise 1: Kerala Board-2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Kerala Board-2018 with Hints & Solutions
Zinc piece and zinc powder are taken in two test tubes and equal amount of dil. is added.
(a) In which test tube does the reaction proceed faster?
(b) Give reason.
(c) Give an instance in daily life, where such condition is made use.
What is the gas liberated when the metals react with dilute acids?
Electrolysis of molten Sodium chloride is conducted.
Which are the ions present in the molten sodium chloride?
Electrolysis of molten Sodium chloride is conducted.
Which is the gas liberated at the positive electrode during electrolysis.
Electrolysis of molten Sodium chloride is conducted.
Write the chemical equation of the reaction taking place at the cathode.
Some materials are given below:
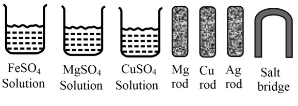
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
Some materials are given below:
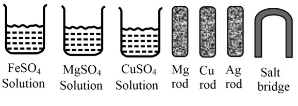
Which is the anode of this cell?
Some materials are given below:
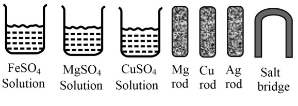
Write the chemical equation fo the reaction taking place at the cathode.
